How to Know Which Resonance Structure Is Best
The structure with a terminal oxygen atom best satisfies the criteria for the most stable distribution of formal charge. 3 The same atoms connected together.
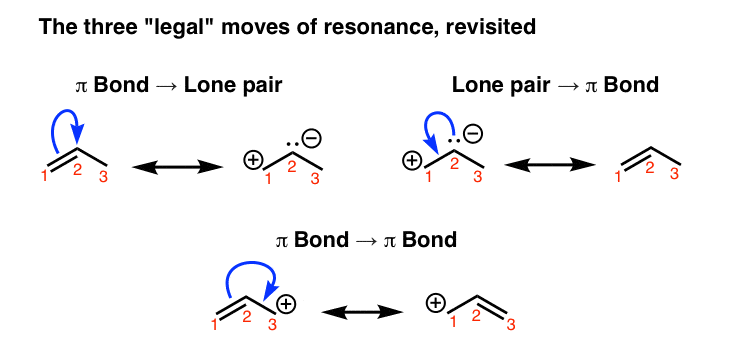
Resonance Structures 4 Rules On How To Evaluate Them With Practice
This molecule has two extra electrons.

. It has minimum charge separation ie. No like charges are located on adjacent atoms. Step 1 Calculate the total number of valence electrons from each atom.
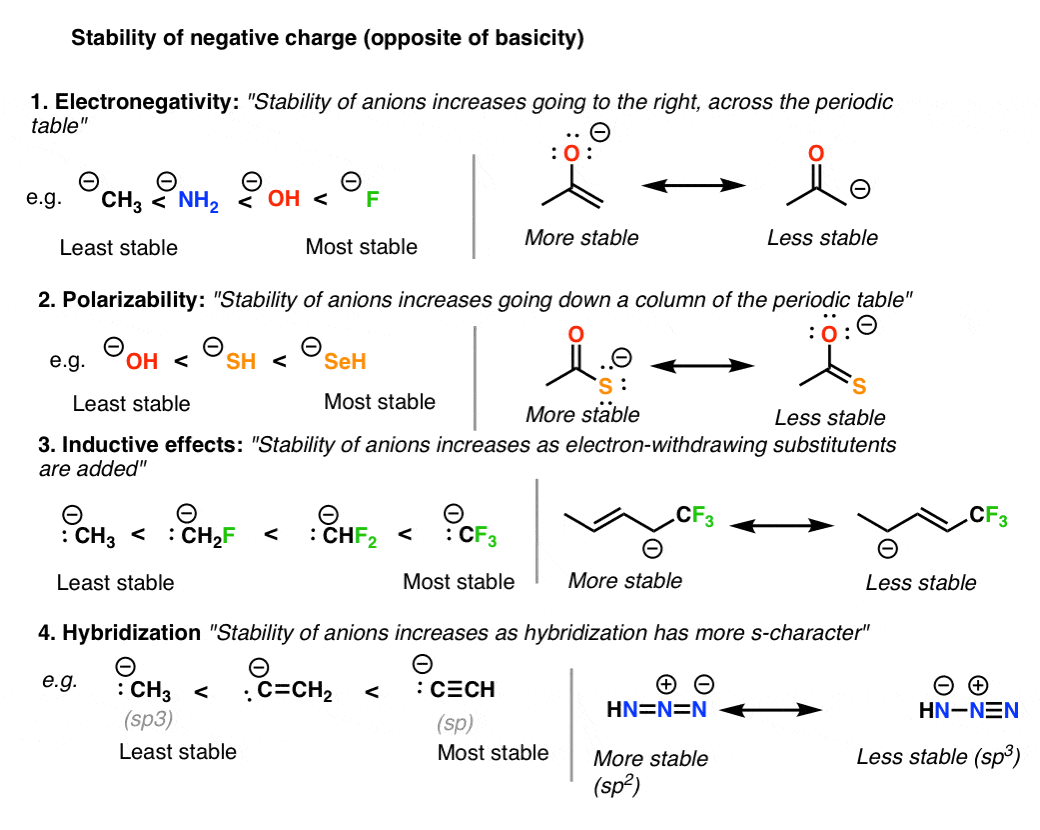
As another hint. Resonance forms with negative formal charge or most electronegative atoms are favored. Again experiments show that all three CO bonds.
118 pm and 136 pm. You need to recognize what each atom generally looks like in an uncharged state. O-NO and ON-O two possibilities Right here the.
Step 2 When there is more than one type of atom keep the least electronegative or metallic atom as the central atom. But to identify each resonance structures it is good to show arrows. 1 The same molecular formulas.
Add all the electron pairs into the structure it might give you an idea. A molecule or polyatomic ion that has multiple Lewis structures because bonding can be. The answer to this question depends on how you define the word best The lewis structure with formal charges closest to 0 is best in the sense that makes a bigger contribution to the resonance hybrid than the other two resonance structures as.
Because resonance structures are the same molecules they must have. The second structure is formed from moving the double bond electrons onto oxygen creating oxygen with a formal -1 charge and carbon with a formal 1. Each contributing resonance structure can be visualized by drawing a Lewis structure.
To draw all resonance structures take the lewis structure we drawn by using VESPR rule. Sometimes it is impossible to avoid charges so if both resonance structures are charged then the octet rule needs to be considered. Provide the best Lewis structure by drawing in any missing multiple bonds and lone pairs.
The N-O bond lengths in dinitrogen pentoxide have two values. Count the valence electrons in your trial structure 20. In following examples arrows are used to show electrons transformation.
Fewer non-zero formal charges 3. The negative charge resides on a more electronegative atom 4. 2 The same total number of electrons same overall charge.
Now count the valence electrons you actually have available. Since the molecular formula is O 3 we know there are 18 valence electrons oxygen has six valence electrons as 6 x 3 18. A way to know which resonance structure is most favorable is to look at the formal charge.
Atoms in general dont like charges so having no charge is better. Rather the true structure is an approximate intermediate between each of the structures. A resonance structure has a greater importance if.
Rules for determining most representative resonance form Resonance forms with the least number of atoms with non-zero formal charge are preferred. The first structure is just as shown with a double bond between carbon and oxygen. The most stable resonance structure will have negative charges on the most electronegative atoms and positive charges on the least electronegative atoms.
Additionally the structure with the negative charge on the most electronegative atom will be more stable. You do not need to provide the Lewis structure again. The most stable resonance structure will have the smallest possible number of charges.
The resonance structure with a. Resonance structures with charge separation are usually higher in energy than those in which charges can be neutralized unless rule 1 is not obeyed. That is the molecule does not actually go back and forth between these configurations.
It helps to start by drawing a simple lewis dot structure. Atoms lose gain or share electrons in order to have a full valence shell of eight electrons. The most stable resonance structure will have a full octet on every atom.
Indicate which N-O bond s are 118 pm and which N-O bond s are 136 pm in the bonding framework below. In resonance structures it does not require to show transformation of electrons by arrows. Oxygen is more electronegative so would a pi bond go to the oxygen atom and make that O-.
It contains a greater number of bonds and atom octets 2. After placing all the electrons we will have a double bond and a single bond. Are you struggling with Resonance structures or just dont really get whats going on when you do it.
The most stable structure will have the lowest formal charge making it the most favorable structure. You can draw 3 possible resonance structures for acetone but only 2 are reasonably considered. Although they can differ.
This helps determine which of a few Lewis structures is most correct. Well its just the movement of ele. Draw a new trial structure this time inserting one double bond for each extra pair of electrons.
Sep 21 2015 at 846. Eg 1 is favored over 2. This will help you to construct the Lewis Dot structure on which you will base your resonance structures.
However it is important to note that each of these structures cannot actually be observed in nature. If charge is separated then the best structure is consistent with the electronegativity of the atoms eg a negative charge is best on the most electronegative atom. Resonance forms with low formal charges are favored over high formal charge.
Also the resonance structure you have drawn is no longer considered major Jan. 1 N 2 O 1 17 26 1 18. The trial structure has two more electrons than are available.
To find the resonance structure of ozone we will draw the lewis structure of ozone. Because we can write three identical resonance structures we know that the actual arrangement of electrons in the carbonate ion is the average of the three structures. Carbon atom 4 Oxygen atoms 36 18 For -2 charge 2 Consider the -2 charge at the last step ie.
1 Know each atoms natural state see tip 8.

How To Choose The More Stable Resonance Structure Chemistry Steps

File Sulfate Resonance 2d Png Wikipedia
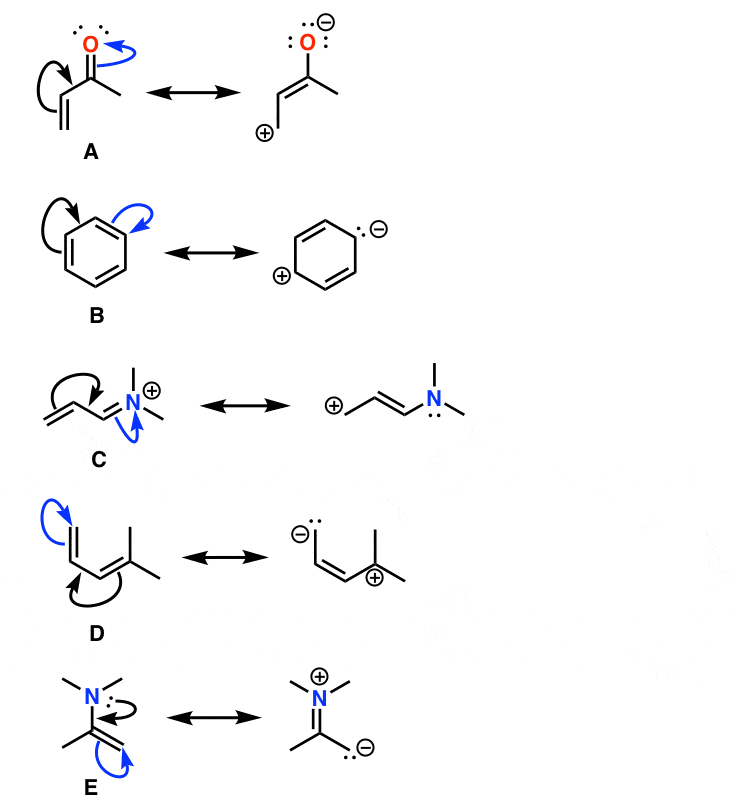
Drawing Resonance Structures 3 Common Mistakes To Avoid

How To Choose The More Stable Resonance Structure Chemistry Steps

How To Choose The More Stable Resonance Structure Chemistry Steps

How To Choose The More Stable Resonance Structure Chemistry Steps
Resonance Structures 4 Rules On How To Evaluate Them With Practice

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry
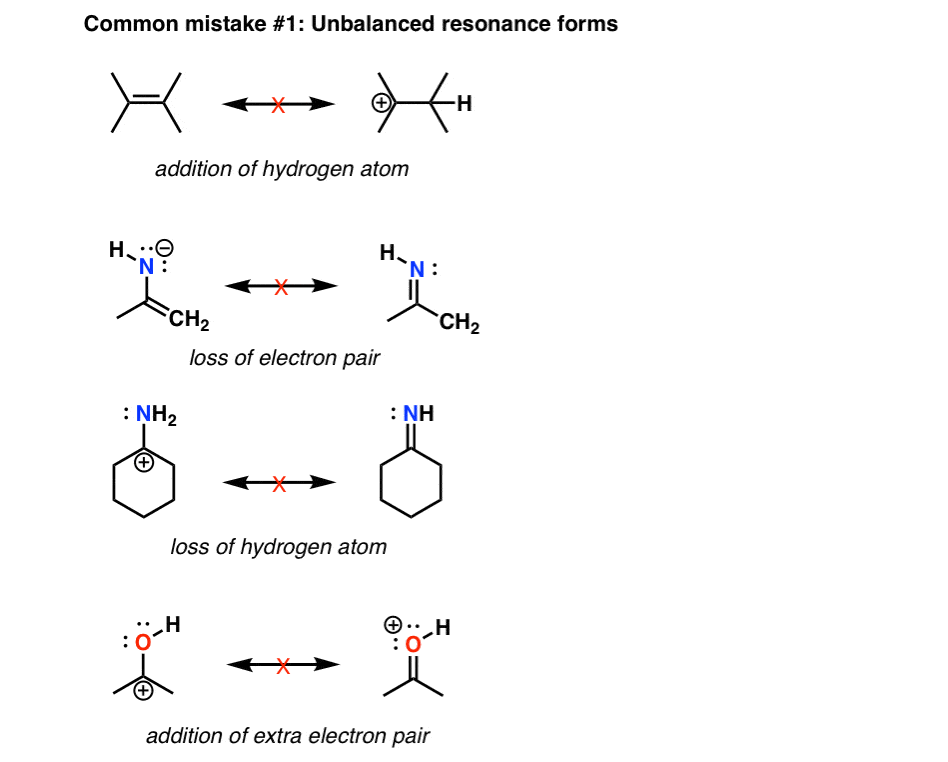
Drawing Resonance Structures 3 Common Mistakes To Avoid

The Predicted Stabilities Of Resonance Contributors Mcc Organic Chemistry
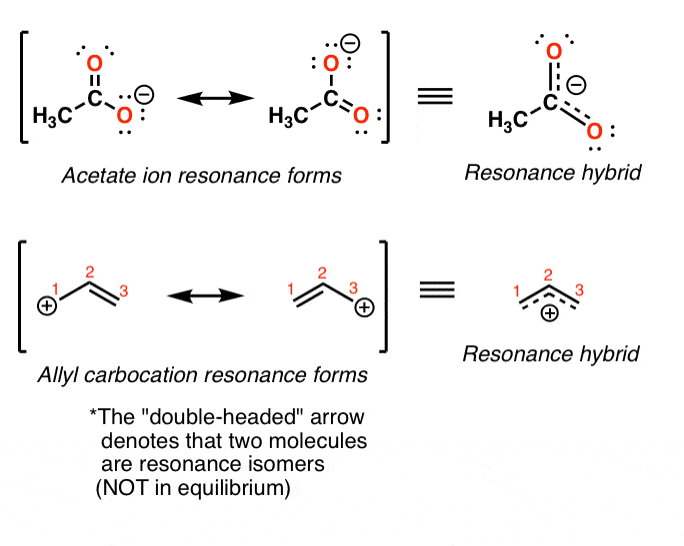
Resonance Structures 4 Rules On How To Evaluate Them With Practice

1 3 Resonance Structures Organic Chemistry I
How To Identify Resonance Structures

How To Choose The More Stable Resonance Structure Chemistry Steps




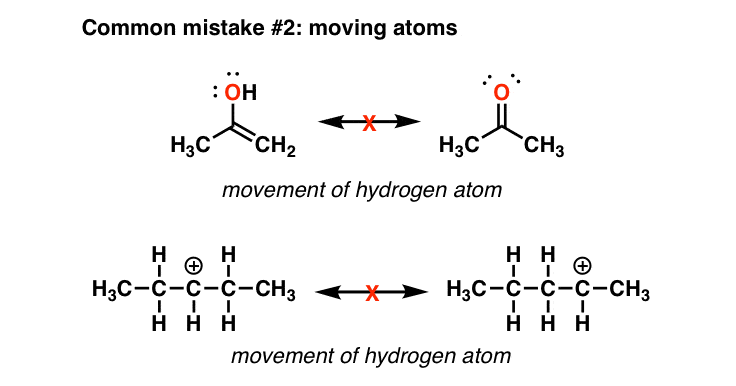
Comments
Post a Comment